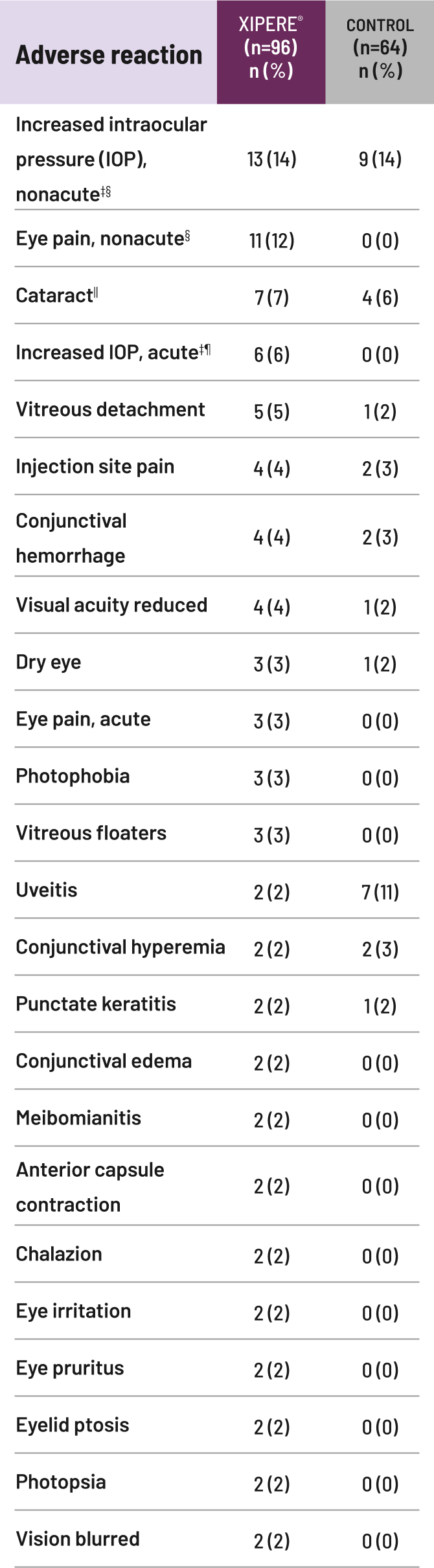
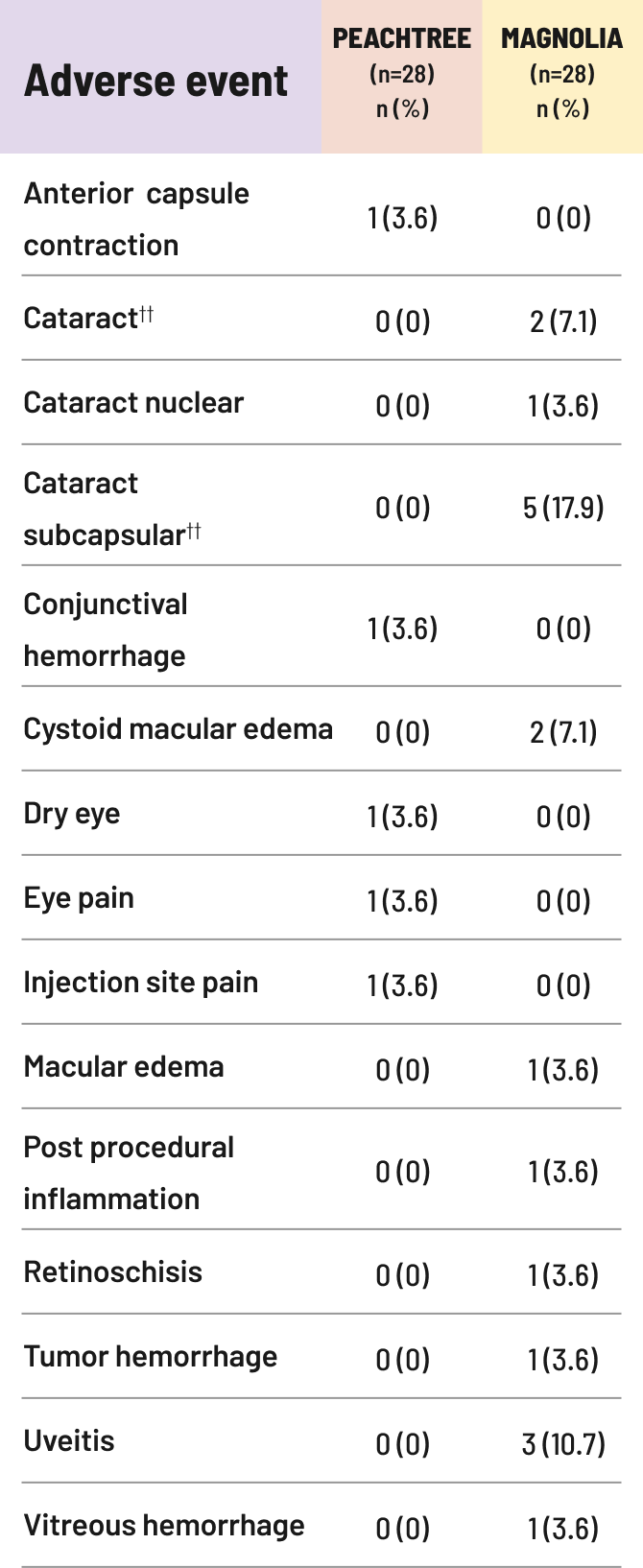
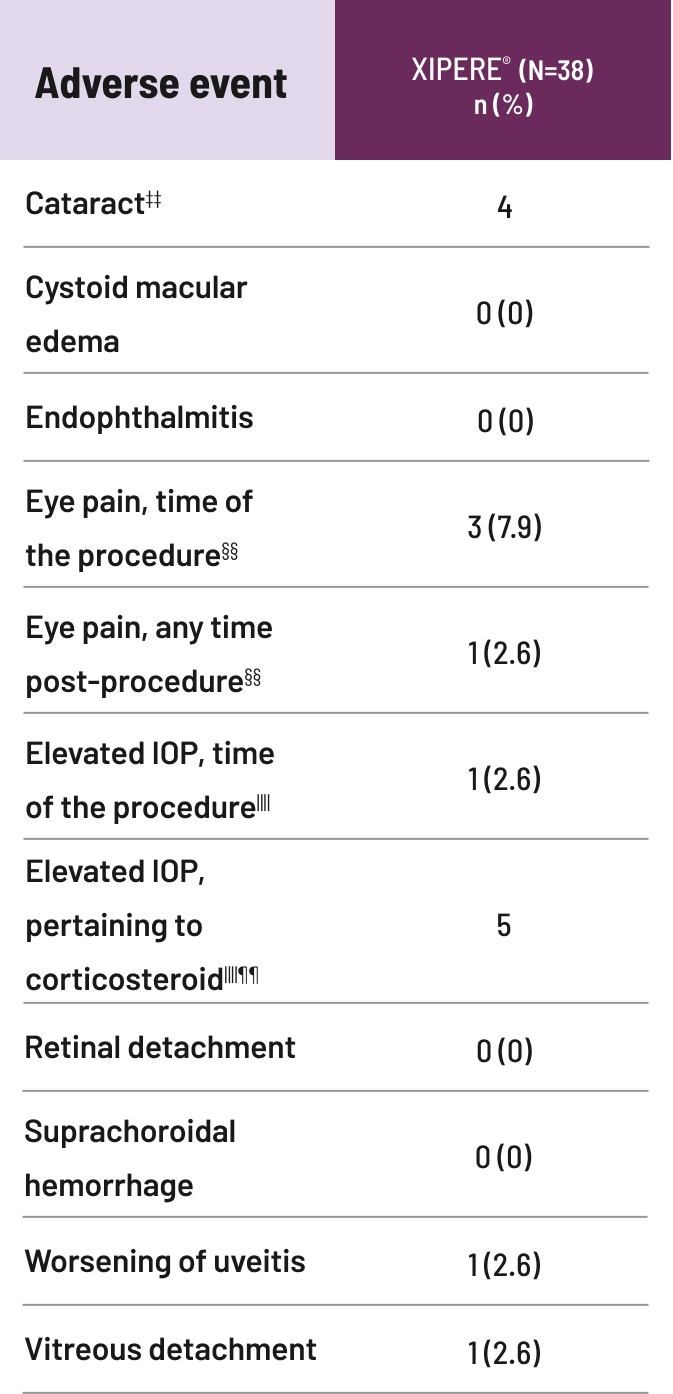
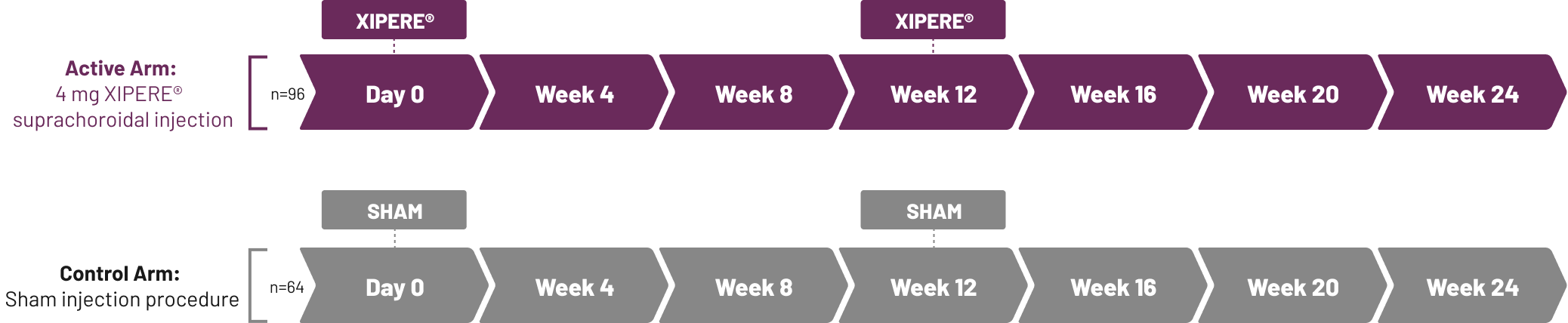
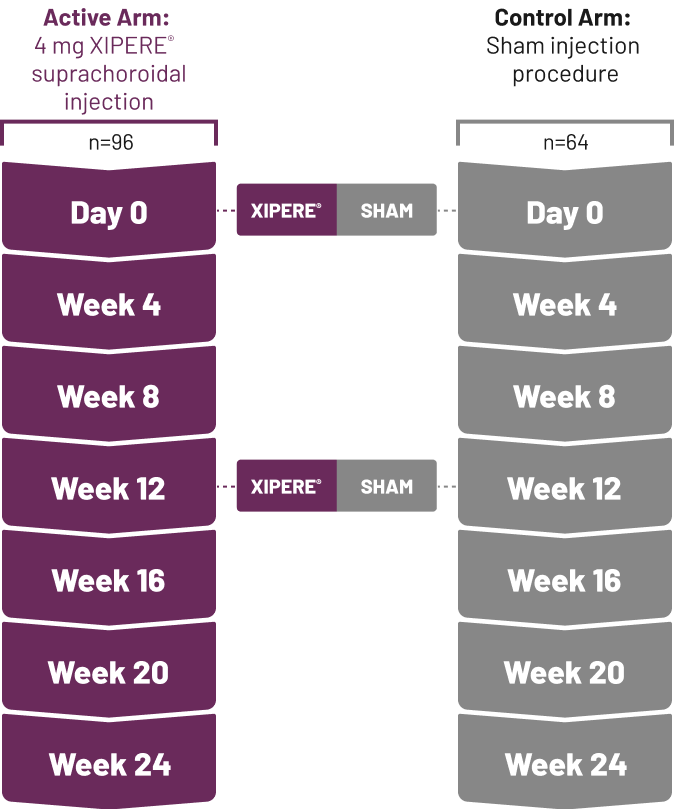
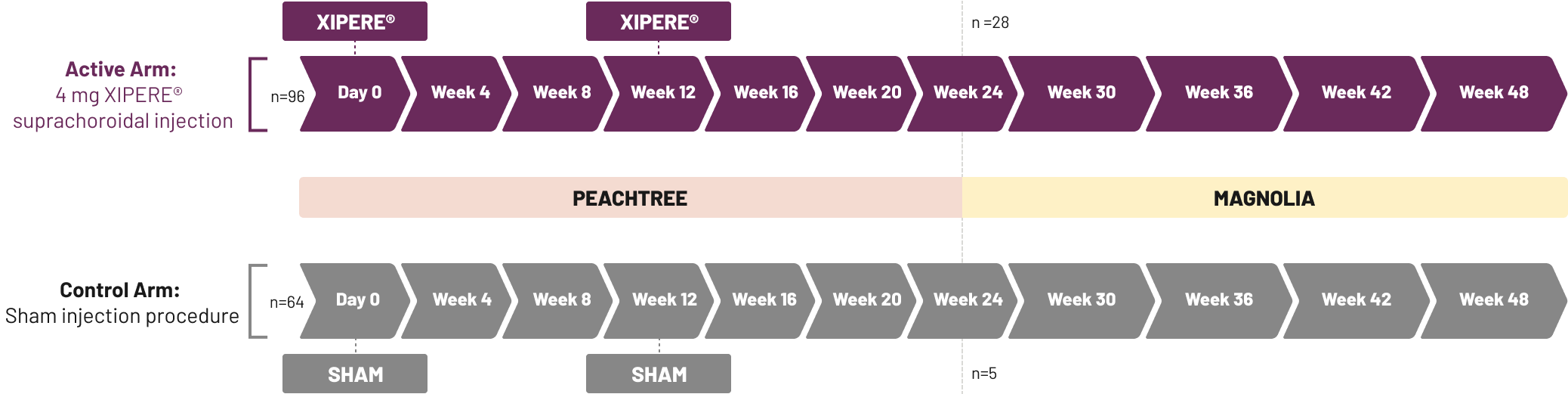
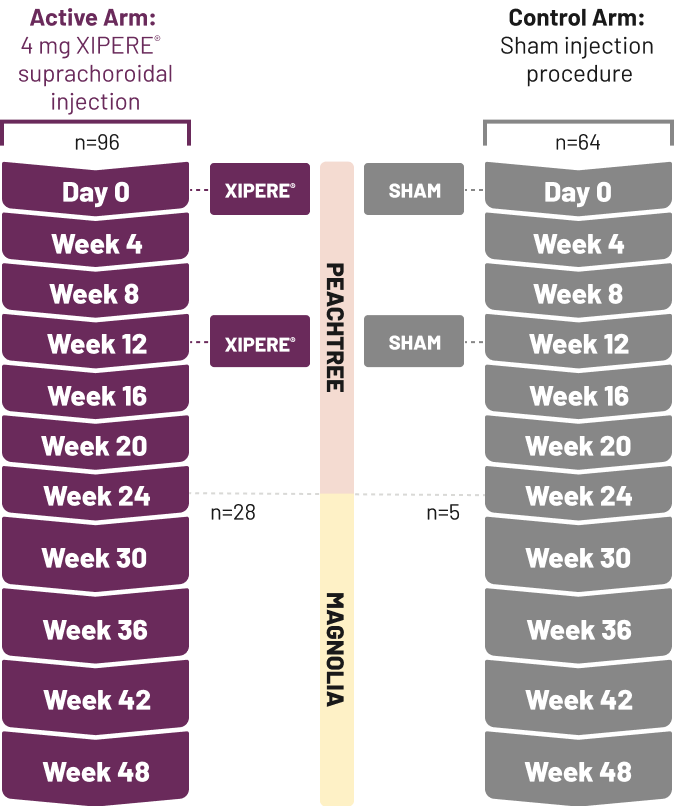
IOP=intraocular pressure.
References
1.Yeh S, Khurana RN, Shah M, et al. Efficacy and safety of suprachoroidal CLS-TA for macular edema secondary to noninfectious uveitis: phase 3 randomized trial. Ophthalmology. 2020;127(7):948-955.
2.Khurana RN, Merrill P, Yeh S, et al. Extension study of the safety and efficacy of CLS-TA for treatment of macular oedema associated with non-infectious uveitis (MAGNOLIA). Br J Ophthalmol. 2022;106(8):1139-1144.
3.Henry CR, Shah M, Barakat MR, et al. Suprachoroidal CLS-TA for non-infectious uveitis: an open-label, safety trial (AZALEA) [published online ahead of print]. Br J Ophthalmol. 2021;0:1-5.
4.Singer M, et al. Presented at: 57th Annual Retina Society Scientific Meeting. September 11-15, 2024; Lisbon, Portugal.
5.XIPERE® [prescribing information]. Alpharetta, GA: Clearside Biomedical, Inc.
6.Data on file. Clearside Biomedical, Inc.